Here we explore the fascinating Classification of chromatography, from adsorption to affinity chromatography. Discover the science behind separation and how these techniques impact medicine, food safety, and scientific breakthroughs. Join us on a chromatographic journey that goes beyond colors, delving into the intricate details of each classification. Dive into the diverse realm of chromatography with us!
What is Chromatography?
Chromatography is the Analytical Laboratory technique or physical method or process in which substances or compounds or components or solutes or sample mixtures are separated to purify or identify from a sample mixture or compound for Qualitative and Quantitative analysis. This type of separation process or method or technique is known or called Chromatography
Derivation or Meaning of Chromatography?
Chromatography word is derived (obtain come start) from the Greek word “Chroma” which means color and “Graphain” which means writing. Thus chromatography means “Color writing”
History of Chromatography
- In ancient times chromatography was first time developed by the Russian botanist Mikhail Tsvet in the early 19th century (1900-01). He used this technique or method to separate plant pigments into different bands by passing through a column filled with calcium carbonate (also known as Limestone (formula CaCo3).
- A few years later in 1906, Mikhail and his co-workers used the (AC) chromatography technique or method for the separation of the plant’s pigments such as Chlorophyll ( which gives the Green Color), Xanthophyll ( which gives the Yellow color) and Carotene (Which gives the Orange color).
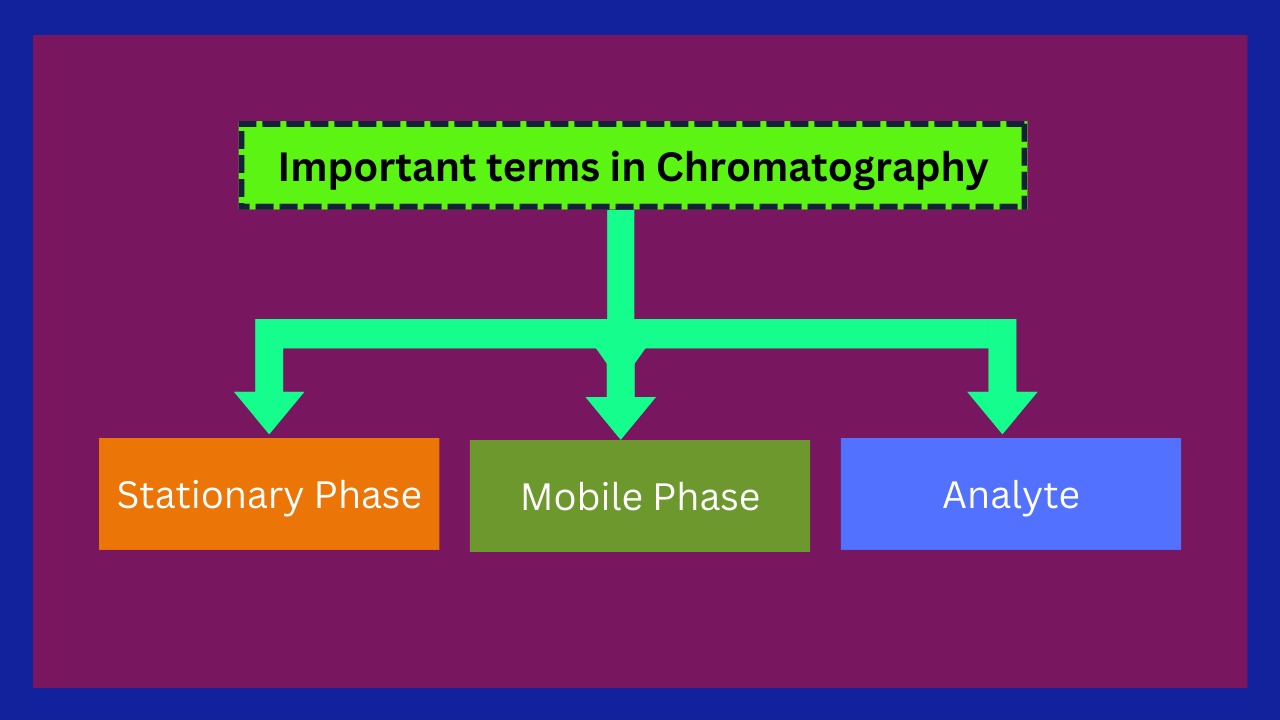
Important terms in Chromatography
The three most important terms that are involved in every type of chromatography are given below:
| 1. Stationary phase | 2. Mobile phase | 3. Analyte |
- The Stationary Phase(s.p) in chromatography is the one that does not move (It means that s.p is fixed) with the sample.
- The Mobile Phase(m.p) in chromatography moves (It means that m.p is not fixed) with the sample.
- Analyte the substance that is to be separated/split during the Chromatography.
Classification of Chromatography
Chromatography is classified into such bases as:
- Principle or Mechanism of the separation
- Shape or Geometry of the Chromatographic bed
- The Polarity of the Phases
- Identification, Purification, Isolation, Quantification
- Nature of Phases (Stationary phase and Mobile phase)
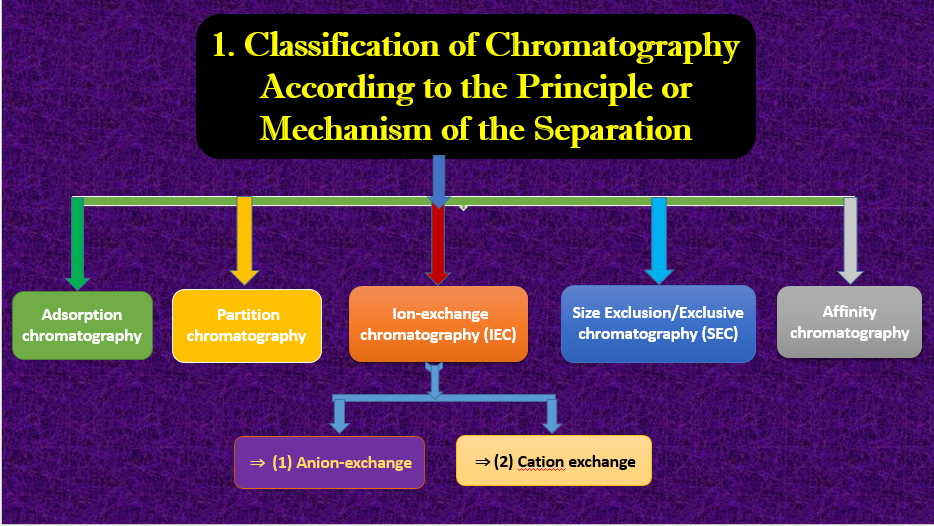
1. Principle or Mechanism of the Separation
According to the separation, chromatography is classified into different types or classes such are given below:
- Adsorption chromatography
- Partition chromatography
- Ion-exchange chromatography (IEC)
- Size Exclusion/Exclusive chromatography (SEC)
- Affinity chromatography
1. Adsorption Chromatography:
Adsorption means to “bond or cling (stick چپکنا) to the surface (choosing anything)”. In this type of chromatography technique or process the chemical mixture or compounds are separated or purified according to the adsorption principles. Adsorption chromatography is a valuable and important tool for separating and purifying in Analytical chemistry. In adsorption chromatography (AC) the stationary phase is Solid (mean Adsorbent) and the mobile phase may be Gass or Liquid
Stationary phase (S.P) ⇒ Solid (Adsorbent) Mobile phase ( M.P) ⇒ Gass or Liquid
Colum chromatography
Thin Layer chromatography
Gass solid chromatography
2. Partition Chromatography:
This type of Analytical technique of chromatography is also known or called Liquid chromatography (LC) (Liquid-Liquid chromatography (LLC) or Liquid-Gass chromatography) (LGC). In this type of chromatography, the stationary phase (s.p) remains Liquid but the mobile phase may be Gass or Liquid. Examples of Partition chromatography are GLC, HPLC, Paper chromatography
Stationary phase (S.P) ⇒ Liquid Mobile phase ( M.P) ⇒ Gass or Liquid
Liquid-liquid chromatography
Gass-Liquid chromatography
3. Ion-Exchange Chromatography (IEC):
Ion exchange chromatography is also known as Ion chromatography. In ion exchange chromatography, charged molecules like inorganic ions, polypeptides, and proteins are separated and purified. Such a stationary phase is selected in ion exchange chromatography, attracting the sample molecules with opposite charges. This stationary phase is also known as an Ion exchanger.
This one is about charged particles. Imagine little magnets on molecules. Ion-exchange chromatography helps scientists separate them based on their charges. It’s like sorting magnets – the positive and negative ones go their own way. This is super useful in making medicines and understanding our bodies better.
Classification of Ion exchange chromatography
⇒ (1) Anion-exchange (AE-chromatography)
⇒ (2) Cation exchange (CE-chromatography)
4. Size Exclusion/Exclusive Chromatography (SEC):
Size exclusion chromatography is also known as gel permeable chromatography. In size exclusion chromatography compounds are separated according to their size. Large molecules elute first and small molecules elute later because they exhibit greater diffusion in the mobile phase within the column. The column is filled with gel. This gel acts as a stationary phase in size exclusion chromatography.
Size exclusion chromatography is like giving everyone a ticket to a party, but only certain sizes can enter. Big molecules get a special pass, and smaller ones have a different path. This is used in studying things like our DNA and proteins.
5. Affinity Chromatography:
Affinity chromatography is a chromatography technique in which separation occurs when two molecules interact with each other. This interaction may be between enzymes and substrates, proteins and nucleic acids receptors and ligands, etc
Affinity chromatography is like matchmaking for molecules. It helps molecules find their perfect match and separate from the others. This is vital in making sure medicines are pure and work well.
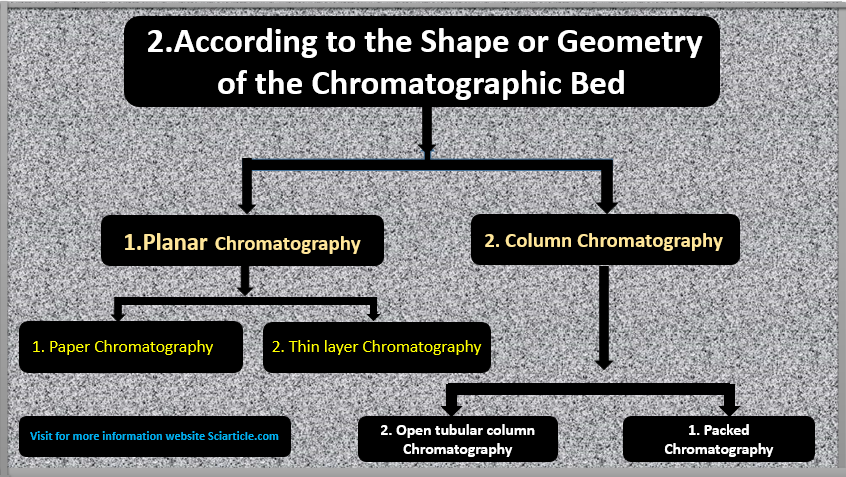
2. Shape or Geometry of the Chromatographic Bed
(A) Planar Chromatography:
This type of Analytical chromatography method in which the stationary phase or chromatographic bed takes place on the plane or flatbed. It has two main types or classes that are given below:
- Paper chromatography
- Thin layer chromatography
(B) Column Chromatography may be ⇒
- Packed Chromatography
- Open tubular column chromatography
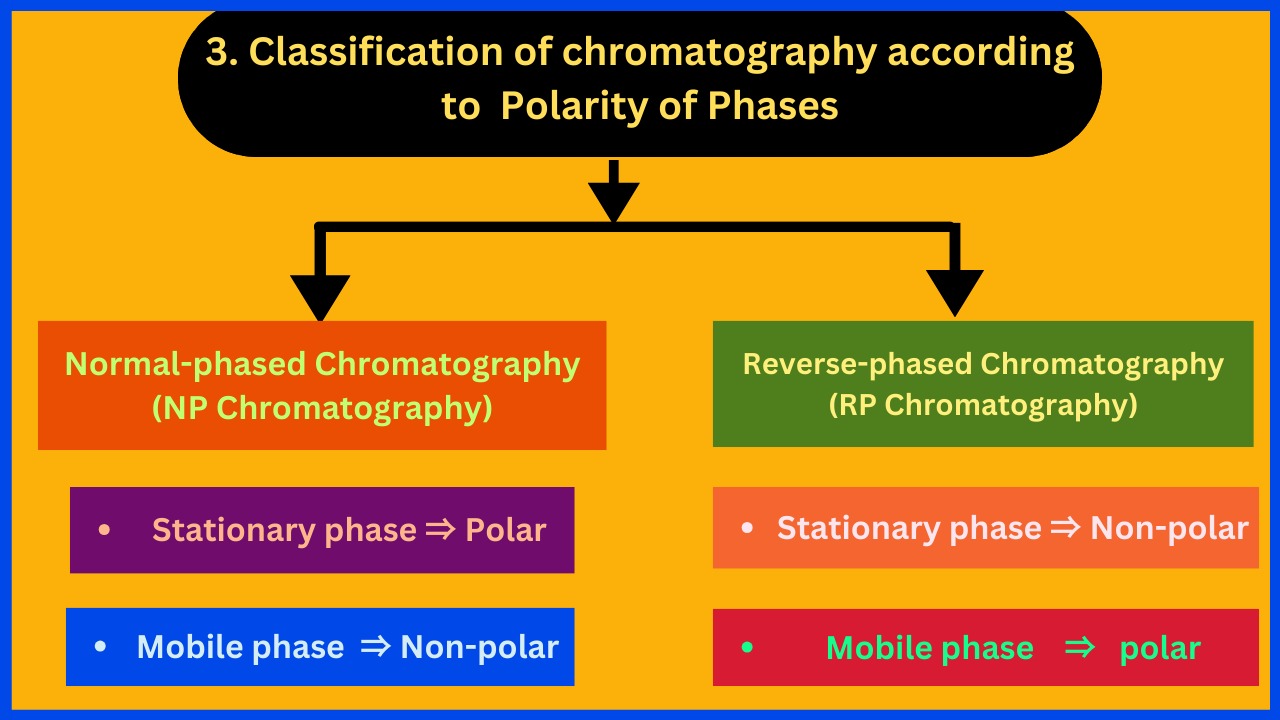
3. Polarity of Phases
According to the polarity of phases, chromatography is classified into two types:
1. Normal-phased Chromatography (NP Chromatography):
Stationary phase ⇒ Polar Mobile phase ⇒ Non-polar
2. Reverse-phased Chromatography (RP Chromatography):
Stationary phase ⇒ Non-polar Mobile phase ⇒ polar
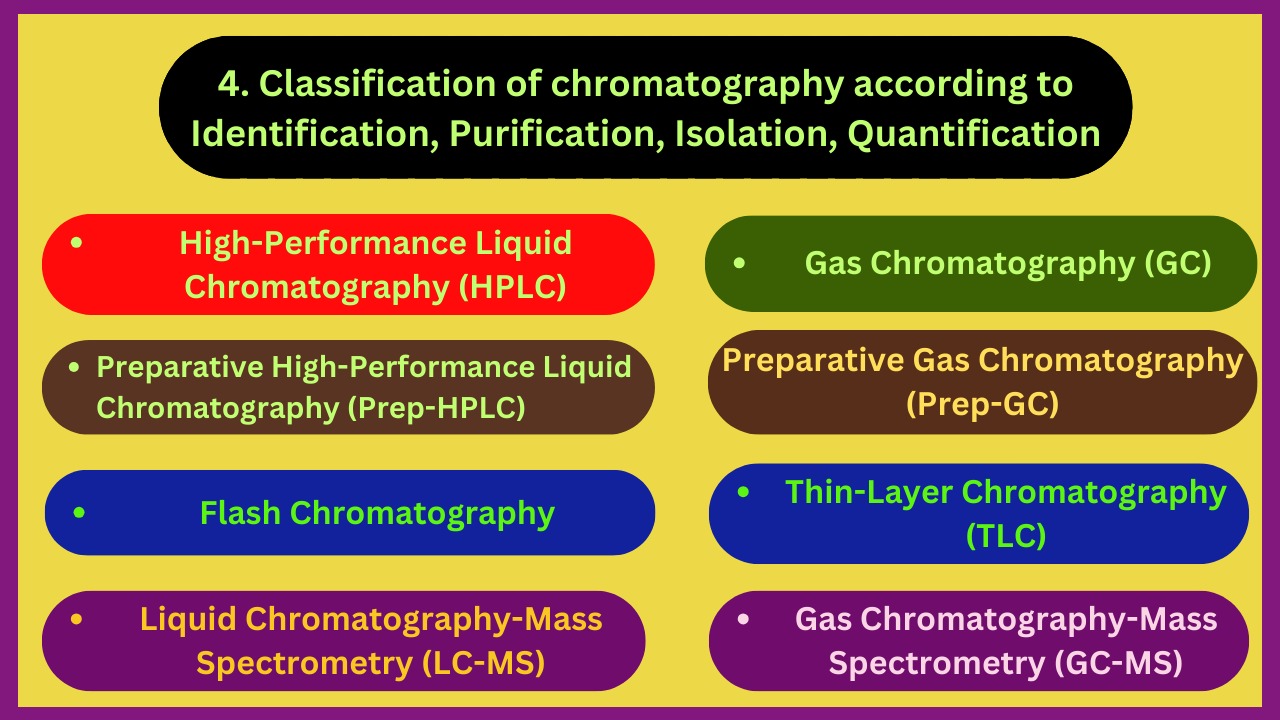
4. Identification, Purification, Isolation, Quantification
- Paper chromatography (PC)
- Gass chromatography (GC)
- High-Performance Liquid chromatography (HPLC)
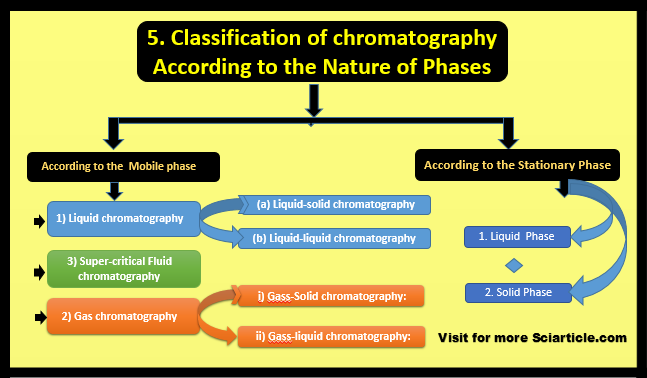
5. Nature of Phases
According to the Nature of Phases, chromatography is classified into two types which are given below:
- Stationary phase
- Mobile phase
Stationary Phase
When we talk about the classification of chromatography according to the Stationary phase (S.P) may be Solid or Liquid
-
-
- Solid Phase
- Liquid Phase
-
Mobile Phase
The Mobile phase which may be Liquid or Gas then chromatography is classified into different types that are given below:
- Liquid Chromatography
- Super-critical Fluid
- Gas Chromatography
Why Chromatography Matters:
Chromatography isn’t just a fancy word for scientists; it’s a super useful tool that’s all around us. Imagine it as a superhero ensuring our medicines are safe, our food is clean, and scientists can uncover new wonders!
Picking the Perfect Chromatography:
Think of chromatography types like different tools in a scientist’s kit. They choose the one that fits the job they’re doing. It’s a bit like selecting the right key for the right door – each type helps unlock the secrets they’re looking for!
Frequently Asked Questions (FAQs)
Is chromatography only used in chemistry?
No, chromatography is used in various fields, including biology, pharmaceuticals, environmental science, and more.
What is the significance of polarity in chromatography?
Polarity plays a crucial role in separating compounds based on their affinity for polar or non-polar phases, influencing the choice of chromatographic methods.
How does preparative chromatography differ from analytical chromatography?
Analytical chromatography focuses on the identification, quantification, and separation of anything while preparative chromatography is geared towards purifying and isolating compounds on a larger scale.
Can gas chromatography be used for non-volatile compounds?
Gas chromatography(GC) is most effective for volatile compounds due to the gaseous nature of the M.P. (mobile phase).
Is liquid chromatography limited to specific types of compounds?
No, It’s not limited to specific types of compounds. So LC can be applied to a wide range of compounds.